Abstract
Background: Despite the many advances in the treatment landscape of relapsed refractory multiple myeloma (RRMM), survival outcomes remain poor for patients with triple class refractory disease (refractory to proteasome inhibitors (PIs), immunomodulatory agents (IMiDs) and anti-CD-38 monoclonal antibodies). While the role of autologous stem cell transplant (ASCT) in the first line therapy is well established, efficacy of ASCT for patients with RRMM in the era of novel therapeutic agents remains unknown. In this single center retrospective analysis, we compared the efficacy and safety outcomes of patients with RRMM treated with daratumumab, pomalidomide, and dexamethasone (DPd) alone versus (vs) DPd followed by ASCT.
Methods: A total of 83 patients with RRMM who were treated and achieved at least partial response (PR) with DPd alone vs DPd followed by ASCT were evaluated by electronic medical records (EMR). DPd was given as daratumumab 16 mg/kg IV or 1800 mg SQ weekly for cycles 1 and 2, every 2 weeks for cycles 3-6, and then every 4 weeks; pomalidomide at 4 mg orally on days 1-21 of a 28-day cycle; and dexamethasone 20 or 40 mg weekly. Twenty-one patients who responded to DPd proceeded with melphalan 140 or 200 mg/m2 followed by ASCT and subsequent maintenance therapy. Responses were evaluated using the international myeloma working group (IMWG) response criteria. Patient and disease characteristics, as well as efficacy and safety outcomes were summarized using descriptive statistics. Kaplan-Meier analyses were used to estimate progression-free (PFS) and overall survival (OS).
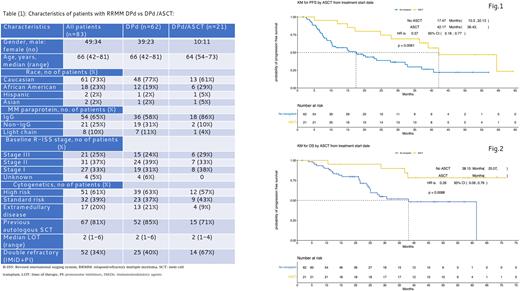
Results: A total of 21/83 (25%) pts with RRMM who achieved at least PR to DPd underwent ASCT (DPd/ASCT) while the remaining 62/83 (75%) continued DPd without ASCT (DPd alone). For the entire patient population, median age was 66 years [IQR 42-81]; 49 (59%) patients were male, 54 (65%) patients had IgG isotype, 21 (25%) patients had R- ISS stage III disease, 51 (61%) patients had high-risk cytogenetics, and 17 (20%) patients had extramedullary disease (Table 1). Patient age, disease stage, and cytogenetic risk profile was balanced between two groups. However, more patients in the DPd/ASCT group when compared to the DPd alone group had not received a prior ASCT (29% vs 15%) or had double refractory disease (67% vs 40%). All patients in this study had achieved at least a PR, though very good partial response (VGPR) or better was observed in 37 (60%) and 18 (86%) patients in the DPd alone and the DPd/ASCT group, respectively. Median PFS was 17.5 months (95%CI 10.3-32.1) with DPd alone vs 42.2 months (95%CI 36.4-NA) in the DPd/ASCT group (p=0.006). Median OS was 38.1 months (95%CI 25.0-NA) for DPd alone group vs not reached in the DPD/ASCT group (p=0.009, Figure 1,2). The most common grade 3 or 4 treatment related adverse events (TRAE) were neutropenia [49 (79%) vs 21 (100%)], anemia [11 (18%) vs 7 (33%)], thrombocytopenia [4 (96%) vs 7 (17%)], neutropenic fever [4 (6%) vs 11 (52%)], hospitalization secondary to TRAE [20 (32%) vs 14 (67%)] with the DPd vs DPd/ASCT groups, respectively. There was no treatment-related mortality in either group.
Conclusion: Our retrospective analysis demonstrates patients who responded to DPd and underwent ASCT afterwards have superior PFS and OS compared to those who received DPd alone. Although DPd followed by ASCT is associated with more cytopenias and its related complications, this treatment appears to be overall safe for many patients with RRMM. These findings should not only be compared with larger multicenter collaborative registry-based data sets but also be tested in a prospective, randomized trial to ascertain the benefit of ASCT beyond the benefit observed by DPd alone.
Disclosures
Hashmi:Sanofi: Consultancy, Speakers Bureau; JANSSEN: Consultancy; KARYOPHARM: Speakers Bureau; GSK: Speakers Bureau; BMS: Consultancy. Mohan:Novartis: Research Funding; Takeda: Research Funding; GSK: Research Funding; BMS/Celgene: Research Funding. Mahmoudjafari:BMS: Membership on an entity's Board of Directors or advisory committees; Merk: Membership on an entity's Board of Directors or advisory committees; Omeros: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees. McGuirk:CRISPR Therapeutics: Consultancy; In8bio, Inc.: Other: IIT Clinical Trial; Nextar: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; Sana: Honoraria; Orca Bio: Research Funding. Atrash:Sanofi: Honoraria, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; GSK: Honoraria, Research Funding; Celgene: Honoraria, Speakers Bureau; Amgen: Research Funding; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal